The Voltaic Pile
Electricity is where Newtonian physics ends and quantum physics begins.
When you rub leather against glass it will build up a static charge that sparks when it touches metal. It works with many materials which happen to be insulators, even clouds rubbing against the ground. But lightening is a difficult form of electricity to work with.
It was made a little easier in 1745 when it was discovered that this static charge could be built up in a jar containing a conductor; first water, eventually metal foil. The Leyden Jar became the primary means of studying electricity for the next half a century. In 1754 the pith-ball electrometer was invented, which allowed measuring this static charge. It dangled two conducting spheres from a wire in a jar, and when the wire touched a Leyden jar the spheres repelled each other. One of the men studying electricity during this time was named Allesandro Volta. His many contributions included improvements to the electrometer, which allowed more precise measurements of the charge in a Leyden Jar. But the charge in these jars was fleeting; once a spark was released by touching a conductor the charge was gone and had to be built back up.
In 1800 Volta demonstrated a device that could supply a steady stream of electricity from a stack of alternating metal disks. This is the discovery that lead to the battery and it was so important that one of the ways we measure electricity is named after him (the Volt, as in voltage).
Twitchy Frogs
Volta’s rival was a man named Luigi Galvani. Galvani had published a paper that described how dissected frogs would twitch when different metals were touched against the nerves. He was convinced that electricity came from the frogs and traveled through the metal, much like static electricity would travel along a metal rod. He even called this Animal Electricity.
Volta disagreed. He believed the frogs twitching was an indicator, like the electrometer, of the existence of electricity. He was convinced that the source came from the metals and the frog just provided a means of conveyance. He soon started using brine-soaked paper instead of frogs and found that he could measure what he called tension across the metals using the same kind of electrometer used to detect static electricity. By stacking alternating disks of metals with brine-soaked paper between them he could multiply the effect. This device became known as the “Voltaic Pile”. We call it a battery, and each pair of metal disks we call a cell.
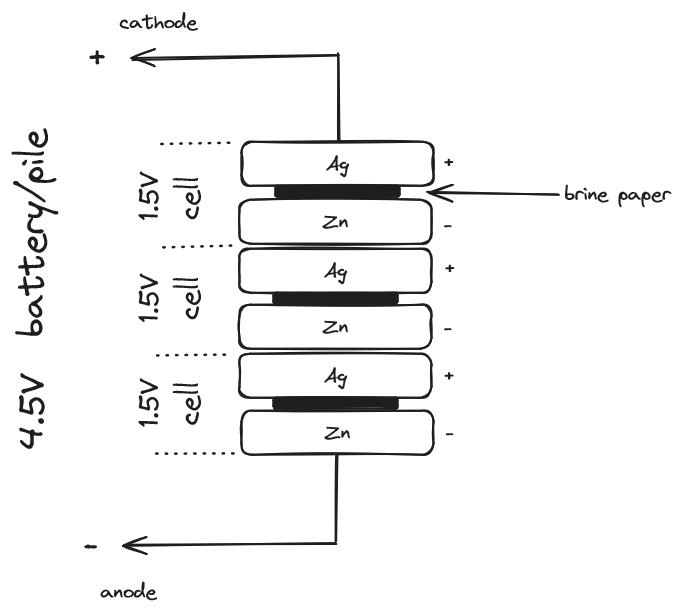
The important difference between a Voltaic pile and a Leyden jar is that this tension quickly dissipates with static electricity (from the jar), but it continues to be present with the pile. This was a different kind of electricity than lightening: direct current (DC), and it could be made to do work.
Chemistry
Volta’s experiment was replicated and studied everywhere. It opened the door to dramatic discoveries in both chemistry and electricity, but it would be chemistry that would ultimately explain how the battery worked. The very names that we use to describe batteries come from those early studies in chemistry by men like Humphry Davy and Michael Faraday.
The key to Volta’s discovery, and what makes the battery work, is the use of electrolytes between the different metal disks. Both the frogs and the salt-brined paper contained electrolytes.
Electrolytes
An electrolyte is a solution that contains ions. Ions are charged particles that can carry their charge within a solution. This charge comes from a difference in protons (positive) and electrons (negative). If an ion has more protons than electrons it has a net-positive charge and is called a cation. If it has more electrons than protons it has a net-negative charge and is called an anion.
As an example consider common table salt, which has one sodium (Na) and one chlorine (Cl) atom combined (NaCl). Sodium has only one outer electron and tends to lose it, while chlorine has seven and tends to gain. When chlorine takes the sodium electron they bind together as sodium chloride.
When salt is added to water, these atoms separate and combine with water (H2O). The sodium forms cations as [Na4(H2O)]+ and the chlorine forms anions as [Cl4(H2O)]-. The chloride ion keeps the electron from the sodium and is negative, the sodium is missing its electron and is positive.
Rather than write the water molecule each time, we use the (aq) subscript to show the element is in an aqueous (water) solution: Na+(aq) and Cl-(aq).
Electrolytes are usually made from either salts or acids in an aqueous ionic solution. The key to an electrolyte is that these ions allow conducting electricity, not by moving electrons, but by moving whole ions. In other words, they affect electricity without being electricity.
The Redox Reaction
A redox reaction occurs when a substance transfers one or more electrons to another substance resulting in a net change of their oxidation states. The substance that loses electrons is said to be oxidized. The substance that gains electrons is said to be reduced. The term “oxidation” comes from the time when this process was thought to only occur with oxygen, but it’s more general than that. The reduction and oxidation always go together, and the full reaction is called a redox reaction.
The silver (Ag) and zinc (Zn) in Volta’s pile can undergo a redox reaction, the extra electron of zinc moves to the silver. When the solid silver is placed in water it produces Ag+(aq) ions; zinc produces Zn2+(aq) ions, along with the solids that remain in the water.
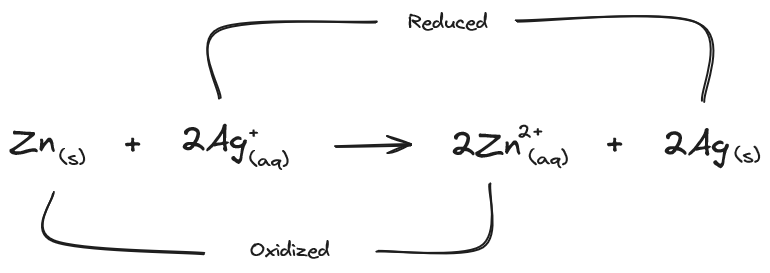
The zinc solid gives it’s electrons to the silver ions to produce solid silver and zinc ions. Heat is released and the reaction soon stops because the charges build up on the solids. What’s needed is a way to split the oxidation and reduction reactions so that the electrons flow out of the solid and into a wire/circuit.
The Galvanic/Voltaic Cell
The key to harnessing the redox reaction between metals is to split the oxidation and reduction into separate halves. This is done by putting an electrolyte between them. The result is known as a Galvanic (for Galvani) or Voltaic (for Volta) cell.
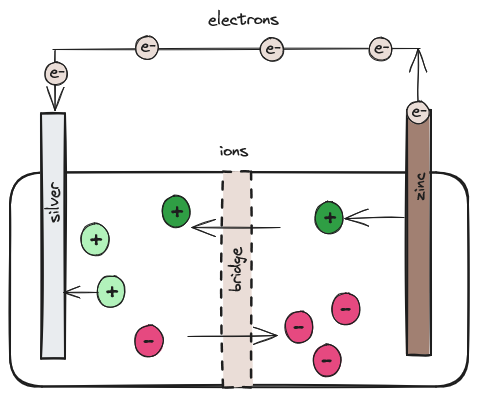
In such a cell the solids are called electrodes; the positive electrode is the cathode (silver in our case) and the negative electrode is the anode (zinc in our case). The solids are placed in a glass jar with an electrolyte. The specific electrolyte doesn’t really matter as long as the ions do not act chemically with either the zinc or silver. The electrolyte is providing ions to balance the electrical charge within the cell. This prevents the electrons from moving directly from the zinc to the silver from within the cell. They stay on the zinc, providing the tension we now call voltage between the electrodes. The electrons find an easier path moving out of the cell along a wire from the zinc anode to the silver cathode, rather than pushing through the charged ions of the electrolyte.
Remember there are two kinds of ions in the solution: the reactive ions from the silver and zinc, and the non-reactive electrolyte ions. The electrolyte ions are critical for sustaining the electron flow. As the electrons leave the zinc anode the remaining zinc atom becomes a zinc ion in the solution; the zinc solid is eventually used up. As the electrons flow into the silver cathode they react with the silver ions on that side of the cell and those ions become solid silver. The non-reactive electrolyte ions move within the cell to maintain the electrical balance. There is often a porous barrier (the bridge), like paper or even unglazed ceramic between the two halves of the cell. This allows the ions to flow through but keeps the solutions on either side from mixing. What’s important is that the electrons only move from solid to solid through the wire; electrons don’t cross the bridge.
For these metals, the voltage across the anode and cathode will measure approximately 1.5V. Most metal combinations will range between 1V and 2V.
Batteries
A battery is a combination of two or more cells, with the anode/cathode of one connected with the cathode/anode of the next. When cells are connected in this way (in series) their voltages are added together. The schematic symbol for a cell is a short line (the anode) next to a longer line (the cathode). So the electrical symbol for a battery is usually two of these connected together (regardless of how many cells are actually in the battery).
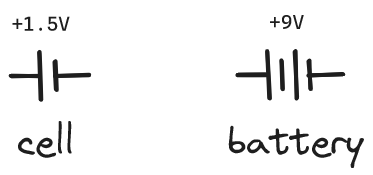
Early batteries looked a lot like our jar cell above, but lots of them connected together. For example, the Grove Cell used a glass jar with a zinc cylinder. Inside the cylinder was an unglazed ceramic cup, and in the cup was another cylinder of platinum. The outer jar was filled with sulfuric acid, the cup with nitric acid. The resulting cell produced a voltage of 1.9V. Many of these connected together could produce voltages of 60-100V. Unfortunately it also gave off a poisonous gas (nitrogen dioxide), but it was popular from 1840-1860. This kind of cell is also called a “wet-cell” because it uses liquid which must not be spilled.
Most modern batteries are composed of “dry-cells”, they don’t use liquid but a paste. When you buy a pack of AA alkaline battery cells in the store they are sealed and don’t contain any liquid. The anode for these batteries is also zinc, but the cathode is manganese, in the form of manganese dioxide paste. The zinc is in the form of powder in a gel of potassium hydroxide (the electrolyte). The two are separated by a cardboard tube.
Electricity
We take for granted that electricity is everywhere and always available. This is a very recent thing. Volta’s battery is an excellent milepost for marking the beginning of the study of electricity and chemistry. In just a few short years it would lead to some foundational theories in electromagnetics, physics and chemistry.